Chemists uncover the buildings of open and closed states of the channel, which might assist the event of antiviral medication to scale back irritation.
MIT researchers have found the open construction of the SARS-CoV-2 E channel, complementing their earlier findings on its closed state. This analysis might help in growing antiviral medication to dam the channel and scale back irritation in COVID-19.
Understanding the SARS-CoV-2 E Channel
The genome of the SARS-CoV-2 virus encodes 29 proteins, considered one of which is an ion channel referred to as E. This channel, which transports protons and calcium ions, induces contaminated cells to launch an inflammatory response that damages tissues and contributes to the signs of COVID-19.
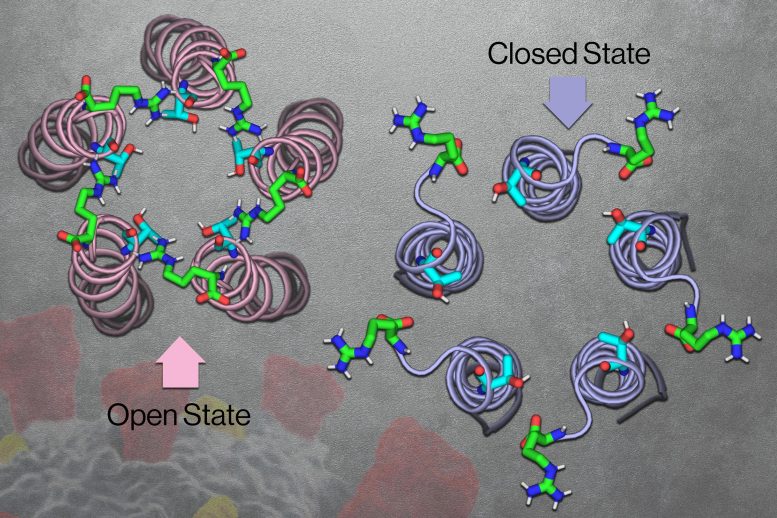
MIT chemists discovered that the SARS-CoV-2 E protein, which acts as an ion channel, has a broad opening on the backside when within the closed state and a narrower opening within the open state. Credit score: Courtesy of the researchers, MIT Information, and iStock
Analysis Advances
“The E channel is an antiviral drug goal. If you happen to can cease the channel from sending calcium into the cytoplasm, then you will have a solution to scale back the cytotoxic results of the virus,” says Mei Hong, an MIT professor of chemistry and the senior writer of the examine.
MIT postdoc Joao Medeiros-Silva is the lead writer of the examine, which was just lately printed within the journal Science Advances. MIT postdocs Aurelio Dregni and Pu Duan and graduate scholar Noah Somberg are additionally authors of the paper.
Investigating Protein Constructions
Hong has in depth expertise in learning the buildings of proteins which can be embedded in cell membranes, so when the COVID-19 pandemic started in 2020, she turned her consideration to the coronavirus E channel.
When SARS-CoV-2 infects cells, the E channel embeds itself contained in the membrane that surrounds a mobile organelle referred to as the ER-Golgi intermediate compartment (ERGIC). The ERGIC inside has a excessive focus of protons and calcium ions, which the E channel transports out of ERGIC and into the cell cytoplasm. That inflow of protons and calcium results in the formation of multiprotein complexes referred to as inflammasomes, which induce irritation.
Structural Insights and Implications
Revealing Atomic-Stage Constructions
To review membrane-embedded proteins similar to ion channels, Hong has developed methods that use nuclear magnetic resonance (NMR) spectroscopy to disclose the atomic-level buildings of these proteins. In earlier work, her lab used these methods to find the construction of an influenza protein referred to as the M2 proton channel, which, just like the coronavirus E protein, consists of a bundle of a number of helical proteins.
Early within the pandemic, Hong’s lab used NMR to investigate the construction of the coronavirus E channel at impartial pH. The ensuing construction, reported in 2020, consisted of 5 helices tightly bundled collectively in what seemed to be the closed state of the channel.
“By 2020, we had matured all of the NMR applied sciences to resolve the construction of this sort of alpha-helical bundles within the membrane, so we have been capable of remedy the closed E construction in about six months,” Hong says.
As soon as they established the closed construction, the researchers got down to decide the construction of the open state of the channel. To induce the channel to take the open conformation, the researchers uncovered it to a extra acidic surroundings, together with increased calcium ion ranges. They discovered that beneath these situations, the highest opening of the channel (the half that will lengthen into the ERGIC) grew to become wider and coated with water molecules. That coating of water makes the channel extra inviting for ions to enter.
That pore opening additionally accommodates amino acids with hydrophilic aspect chains that dangle from the channel and assist to draw positively charged ions.
Channel Dynamics and Drug Growth
The researchers additionally discovered that whereas the closed channel has a really slender opening on the prime and a broader opening on the backside, the open state is the other: broader on the prime and narrower on the backside. The opening on the backside additionally accommodates hydrophilic amino acids that assist draw ions via a slender “hydrophobic gate” in the midst of the channel, permitting the ions to finally exit into the cytoplasm.
Close to the hydrophobic gate, the researchers additionally found a decent “belt,” which consists of three copies of phenylalanine, an amino acid with an fragrant aspect chain. Relying on how these phenylalanines are organized, the aspect chains can both lengthen into the channel to dam it or swing open to permit ions to move via.
“We predict the aspect chain conformation of those three repeatedly spaced phenylalanine residues performs an vital function in regulating the closed and open state,” Hong says.
Future Analysis Instructions
Potential for Antiviral Therapies
Earlier analysis has proven that when SARS-CoV-2 viruses are mutated in order that they don’t produce the E channel, the viruses generate a lot much less irritation and trigger much less harm to host cells.
Working with collaborators on the College of California at San Francisco, Hong is now growing molecules that would bind to the E channel and stop ions from touring via it, in hopes of producing antiviral medication that would cut back the irritation produced by SARS-CoV-2.
Her lab can also be planning to analyze how mutations in subsequent variants of SARS-CoV-2 would possibly have an effect on the construction and performance of the E channel. Within the Omicron variant, one of many hydrophilic, or polar, amino acids discovered within the pore opening is mutated to a hydrophobic amino acid referred to as isoleucine.
“The E variant in Omicron is one thing we wish to examine subsequent,” Hong says. “We will make a mutant and see how disruption of that polar community adjustments the structural and dynamical side of this protein.”
Reference: “Atomic construction of the open SARS-CoV-2 E viroporin” by João Medeiros-Silva, Aurelio J. Dregni, Noah H. Somberg, Pu Duan and Mei Hong, 13 October 2023, Science Advances.
DOI: 10.1126/sciadv.adi9007
The analysis was funded by the Nationwide Institutes of Well being and the MIT Faculty of Science Sloan Fund.