A brand new drug, donanemab, is being hailed as a turning level within the combat towards Alzheimer’s, after a worldwide trial confirms it slows cognitive decline.
The antibody medication helps within the early levels of the illness by clearing a protein that builds up within the brains of individuals with any such dementia.
Though not a remedy, charities say the leads to the journal JAMA mark a brand new period the place Alzheimer’s could be handled.
The UK’s medicine watchdog has began assessing it for potential NHS use.
The drug works in Alzheimer’s illness, not in different kinds of dementia, resembling vascular dementia.
Within the trials, it seems to have slowed the tempo of the illness by a couple of third, permitting individuals to retain extra of their day-to-day lives and duties, resembling making meals and having fun with a interest.
Mike Colley, who’s 80, is considered one of only some dozen sufferers within the UK to participate within the world trial. He and his household spoke solely with the BBC.
Mike will get an infusion every month at a clinic in London and says he’s “one of many luckiest individuals you’ll ever meet”.

Mike Colley (L) along with his son Mark
Mike and his household observed he was having issues with reminiscence and decision-making, not lengthy earlier than he began on the trial.
His son, Mark, mentioned it was very laborious to look at in the beginning: “Seeing him battle with processing info and fixing issues was very laborious. However I feel the decline is reaching a plateau now.”
Mike, who’s from Kent, mentioned: “I really feel extra assured on daily basis.”
Donanemab, made by Eli Lilly, works in the identical manner as lecanemab – developed by firms Eisai and Biogen – which created headlines all over the world when it was confirmed to gradual the illness.
Though extraordinarily promising, these medicine should not risk-free remedies.
Mind swelling was a typical side-effect in as much as a 3rd of sufferers within the donanemab trial. For many, this resolved with out inflicting signs. Nevertheless, two volunteers, and presumably a 3rd, died because of harmful swelling within the mind.
One other antibody Alzheimer’s drug, referred to as aducanumab, was lately rejected by European regulators over security considerations and a scarcity of proof that it was efficient sufficient for sufferers.
What’s dementia and what could be performed about it?
Within the donanemab trial, researchers examined 1,736 individuals aged 60 to 85 with early-stage Alzheimer’s.
Half of them obtained a month-to-month infusion of the therapy and the opposite half got a dummy drug, also called a placebo, over 18 months.
- The drug appears to have a significant profit, at the least for some sufferers
- Those that had earlier illness and fewer mind amyloid at baseline derived higher profit, when it comes to clearance seen on mind scans
- These given the drug additionally retained extra of their day-to-day lives resembling having the ability to focus on present occasions, reply the cellphone or pursue hobbies
- The tempo of the illness, judged by what individuals may nonetheless do day-to-day, was slowed by about 20-30% general – and by 30-40% in a set of sufferers who researchers thought extra more likely to reply
- There have been vital side-effects and sufferers will want to pay attention to dangers of therapy
- Half of sufferers on donanemab had been capable of cease the therapy after a 12 months, as a result of it had cleared ample mind deposits
Amyloid is only one a part of the complicated image of Alzheimer’s, and it’s unclear if the therapy will proceed to make extra distinction over an extended interval, consultants warning.
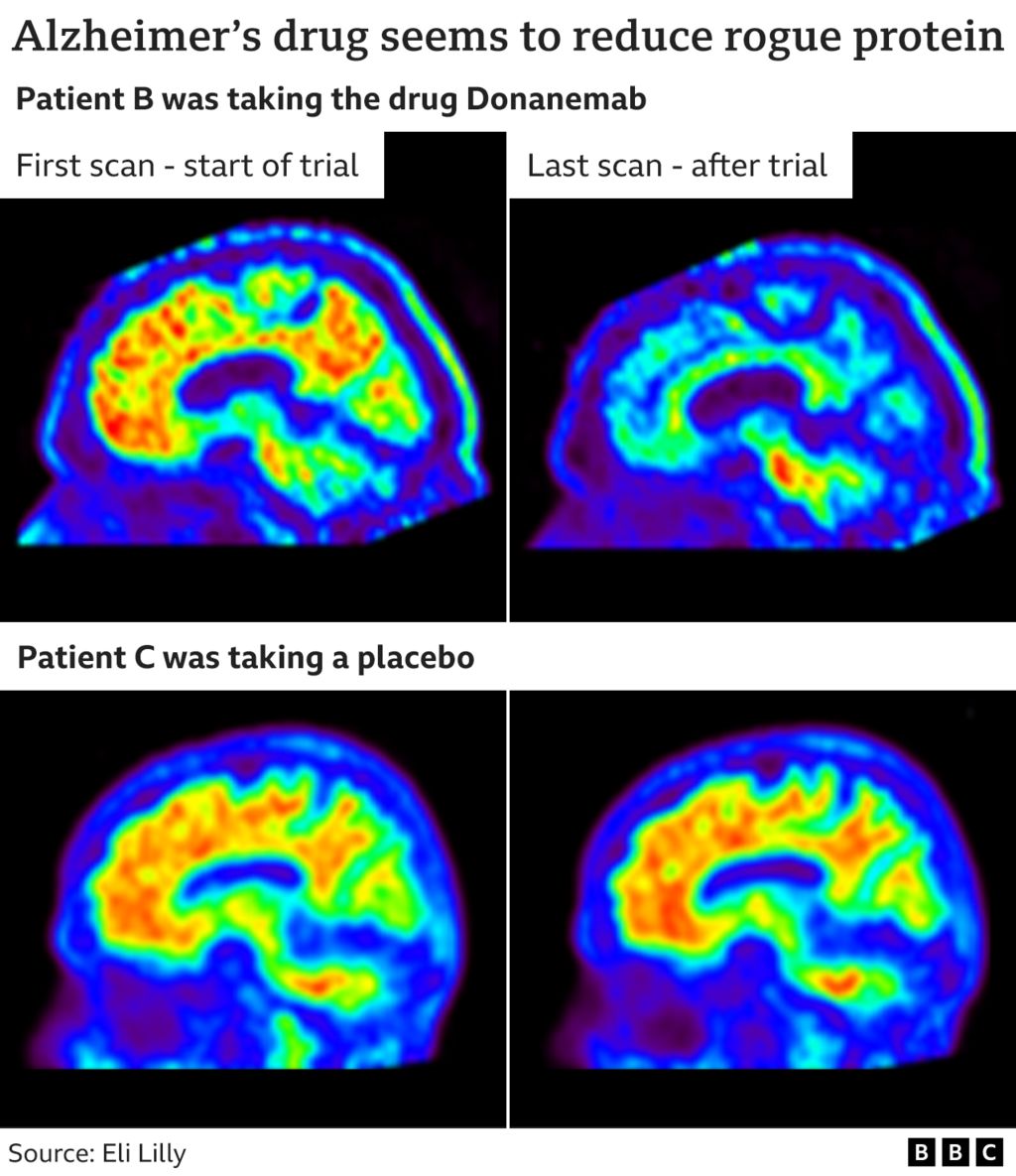
Earlier than and after scans present how the drug cleared deposits (seen in inexperienced, yellow and pink) from the mind
The drug’s results could also be modest, however the outcomes present additional affirmation that eradicating amyloid from the mind could change the course of Alzheimer’s, and assist individuals affected by this devastating illness in the event that they’re handled on the proper time, they are saying.
Prof Giles Hardingham from the UK Dementia Analysis Institute mentioned: “It’s terrific to see these outcomes printed in full right this moment.
“Now we have waited a very long time for Alzheimer’s remedies, so it’s actually encouraging to see tangible progress persevering with to assemble tempo within the discipline.”
Dr Susan Kohlhaas, from Alzheimer’s Analysis UK, mentioned: “At the moment’s announcement marks one other milestone.
“Because of a long time of analysis, the outlook for dementia and its impression on individuals and society is lastly altering, and we’re coming into a brand new period the place Alzheimer’s illness may grow to be treatable.”
Talking to BBC Radio 4’s PM programme, former Prime Minister David Cameron mentioned sources must be put in direction of additional analysis into what he referred to as a “statin for the mind”.
“We wish a tablet that individuals who have the build-up of those proteins within the mind can take on daily basis or each week as a way to clear these proteins out of the mind and subsequently cut back your probabilities of getting a illness that causes dementia,” he mentioned.
Requested if the federal government had been ready to take a position the place wanted to roll out new remedies, Mr Cameron mentioned there was an actual incentive to take action: “We’re a rustic of sixty million individuals, with one million individuals with dementia, a lot of them in very costly residential care settings and so there may be quite a lot of financial savings available from successfully treating individuals….I’m hopeful that our system can ship.”
Lecanemab prices round $27,500 (£21,000) within the US, the place it’s licensed.
It isn’t clear how a lot donanemab could price and the way lengthy it would take to get approval within the UK, however Alzheimer’s consultants mentioned having two medicine would assist promote competitors on worth.
The UK’s drug’s watchdog NICE says it has already began work on its appraisal of donanemab for treating delicate cognitive impairment or delicate dementia attributable to Alzheimer’s illness.
“Our intention is to supply suggestions on its use within the NHS as shut as potential to it receiving its UK licence,” mentioned a spokesperson.
Mike Colley turned 80 in April. At his party, he shocked his household by singing My Method in entrance of 40 visitors.
He instructed BBC Information: “That’s the boldness I’ve now. I’d by no means have performed that even 12 months in the past.”
His son Mark added: “I by no means thought I might see my dad so energetic once more. It was an unbelievable second.”
Dr Emer MacSweeney, guide neuroradiologist and medical director at Re:Cognition Well being, led the trials of donanemab within the UK.
She mentioned: “That is actually vital and one of many greatest breakthroughs.”
The Alzheimer’s Society mentioned: “That is really a turning level within the combat towards Alzheimer’s and science is proving that it’s potential to decelerate the illness.”
Round 720,000 individuals within the UK may probably profit from these rising new Alzheimer’s illness remedies in the event that they’re permitted to be used, however the Alzheimer’s Society mentioned the NHS is “merely not able to ship them”.
Kate Lee, CEO for the charity, mentioned: “Well timed, correct analysis is essential, and at the moment solely 2% of individuals in England and Wales obtain their analysis via the specialist investigations wanted to be eligible for these remedies.
“Alongside this, these rising Alzheimer’s illness medicine require common infusions and monitoring, and the NHS is just not but outfitted to do that at scale.”