By taking goal at a course of frequent throughout many viruses, the drug might sooner or later cease any variety of recognized viruses — and new ones.
t’s about as audacious an concept as yow will discover on the planet of infectious illness: a broad-spectrum antiviral — a “penicillin for viruses.”
The stakes are extremely excessive. The yearly tide of seasonal flu causes three to 5 million extreme circumstances yearly, killing lots of of 1000’s of individuals. The “break-bone fever” of dengue virus threatens roughly half the world’s inhabitants. 350 million individuals at this time live with hepatitis B or C, resulting in thousands and thousands of circumstances of liver illness and most cancers. The HIV and COVID-19 pandemics have already killed tens of thousands and thousands, and viruses from Ebola to chicken flu are continuously threatening future epidemics, too.
All informed, viral infections will account for tens of thousands and thousands of deaths and trillions in financial losses over the approaching a long time. A drug that might deal with all of them — or perhaps a broad class of them — can be big.
Viral infections will account for tens of thousands and thousands of deaths and trillions in financial losses over the approaching a long time. A drug that might deal with all of them — or perhaps a broad class of them — can be big.
Viruses have refined defenses, developed over eons of evolution. When they’re inside cells, it’s usually too late. Once they’re exterior of cells, these tiny invaders are tough to identify. And one favourite drug approach — attacking the biochemical pathways of organisms — doesn’t work on viruses; they aren’t even alive.
“So yeah, it’s a tough downside.”
The New Zealand-based startup Kimer Med, the place Kiessig is the co-founder, chief science officer, and CEO, is taking over the formidable mission of fixing that downside. Impressed by work revealed in 2011, they’re attempting to develop a broad-spectrum antiviral that makes use of a goal in human cells to hopefully assault all kinds of viruses.
The expertise behind it has already failed to achieve traction as soon as, however Kimer is betting that this time — in a post-pandemic world — can be completely different.
An inspiration
The earlier method, referred to as DRACO, and Kimer’s new candidate, referred to as VTose (pronounced “vee-tohs”), each hinge on one thing referred to as double-stranded RNA. However whereas their targets are the identical, VTose makes use of completely different proteins to kind its virus-fighting “toolkit,” as Kiessig places it.
Double-stranded RNA, or dsRNA, is a signature of virus replication in cells, a sign that almost all viral infections create.
The brand new design, VTose, acknowledges the dsRNA and triggers a course of referred to as apoptosis, programmed loss of life, within the contaminated cell — hopefully stopping the an infection in its tracks.
Apoptosis is “a nicely understood course of,” Mark Hickman, a senior scientist with a element of the US Division of Protection’s Chemical and Organic Protection Program, tells Freethink. Hickman and colleagues helped develop comparatively broad-spectrum antivirals towards filoviruses, like Ebola and Marbrug, which culminated within the drug remdesivir (which was later repurposed to battle COVID-19).
Taking goal at dsRNA to make a broad-spectrum antiviral “sounds believable,” Hickman says, with the caveat that extra information, together with medical information, is required.
The dsRNA mechanism has already proven some promise. In 2011, Todd Rider — a polymath with a number of levels from MIT, then affiliated with MIT’s Lincoln Laboratory — and colleagues revealed the preliminary DRACO paper as proof-of-concept.
In these preliminary research, the Rider Institute explains, DRACO was discovered to be efficient towards 18 completely different viruses in 13 completely different sorts of mammalian cells, together with kind 2 dengue virus, H1N1 influenza, and a number of rhinoviruses and coronaviruses, which trigger the frequent chilly. It was additionally discovered to be unhazardous in mice and guarded or rescued them from numerous virus challenges, together with H1N1 influenza.
In August 2021, a yr after its founding, Kimer Med licensed the ultimate DRACO-related patent from MIT and began constructing their very own broad-spectrum antiviral.
The brand new contender
Kimer’s drug is not like most antivirals, which use small molecules. As an alternative, VTose is a big, complicated molecule, constructed from items of proteins which can be current in most of our cells.
Massive and small compounds have completely different benefits and downsides. Whereas they will price extra to develop, Kiessig says, small molecule antivirals are cheaper and simpler to make than giant molecules. However their antiviral talents can minimize each methods. Small molecule compounds have a historical past of being “comparatively poisonous” to wholesome cells, Kiessig says — therefore the costly preliminary growth.
“It requires quite a lot of work, quite a lot of completely different trials — lots of of various compounds, probably — to attempt to discover a unhazardous small molecule that each interferes with the pathway you need it to intrude with,” Kiessig says, “and doesn’t kill the affected person within the course of.”
Massive molecule compounds have a greater security repute, however are tougher and costly to fabricate.
The brand new design, VTose, acknowledges dsRNA and triggers a course of referred to as apoptosis, programmed loss of life, within the contaminated cell — hopefully stopping the an infection in its tracks.
Focusing on the host
Broad-spectrum antivirals fall into two completely different classes: antivirals that concentrate on the viruses straight, and antivirals that concentrate on the cells viruses infect and use for replication. Viruses are extremely various, so discovering a drug that straight works towards a variety of them can be a really tough job.
As an alternative, VTose falls into the latter class, referred to as “host-directed antivirals” (HDAs). An HDA has a couple of advantages that make it a probably ultimate alternative for a broad-spectrum antiviral.
As a result of it’s host-directed, it ought to be tougher for viruses to evolve their means round it — the bane of all antiviral remedies, from COVID-19 to HIV.
“We consider [how VTose works] is way more immune to adaptation by the virus,” Kiessig says. “As a result of what we’re doing is definitely attacking one thing that’s frequent throughout just about all viruses … we’re attacking a fundamental facet of viral replication.”
A broad-spectrum HDA has one other potential profit: it may very well be developed earlier than a brand new virus emerges.
“A pre-existing repertoire of first-line, broad-acting HDAs that may be readily deployed could also be useful in slowing the preliminary viral unfold or in suppressing outbreaks,” Boston College researcher Vipul C. Chitalia and drug discoverer Ali H. Munawar wrote within the Journal of Translational Medication. “Later, HDAs will be complemented with [direct-acting antivirals] and vaccines since their growth hinges on the data of particular viral proteins.”
However to try this, a broad-spectrum antiviral wants a goal that’s frequent throughout many sorts of viruses — throughout viruses that infect completely different sorts of cells, in several methods — and is unlikely for the virus to mutate round. Enter double-stranded RNA.
“It’s precisely the identical type of factor that the innate immune system tries to do.”
RICK KIESSIG
How VTose works
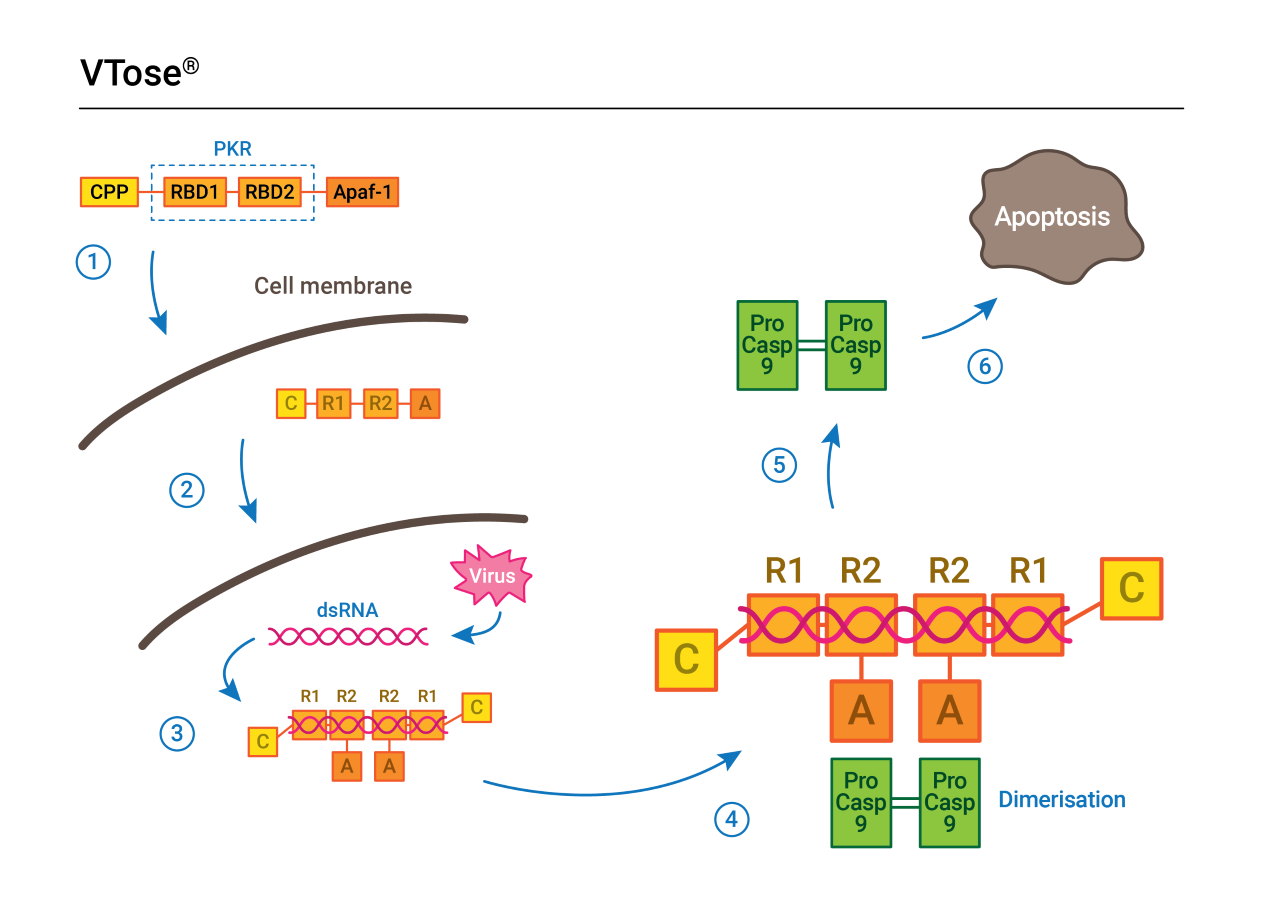
VTose works by taking goal at dsRNA.
In accordance with researchers Y. Grace Chen and Solar Hur, at Yale and Harvard respectively, dsRNA is current within the majority of viral infections. It may possibly come from the viral genome itself, or it may be created within cells throughout viral replication.
“Therefore, practically all organisms have the aptitude of recognizing dsRNA and mounting a response, the first goal of which is to mitigate the potential an infection,” they wrote.
Sadly, viruses are good at what they do and might flummox the efforts of the innate immune system, the primary line of protection in cells that acknowledges dsRNA.
VTose works by recognizing the lengthy strings of dsRNA that happen when a virus tries to duplicate inside a cell. The big molecule then triggers the contaminated cell’s pure suicide sequence — apoptosis — to guard the remainder of the physique.
“It’s precisely the identical type of factor that the innate immune system tries to do,” Kiessig says.
The primary a part of VTose’s course of makes use of the identical protein that cells naturally use to detect viruses, a bit of an enzyme referred to as PKR. When it detects an an infection, VTose then makes use of a part of a protein referred to as Apaf-1, which kicks off a biochemical chain response that leads to apoptosis, killing the cell earlier than the virus can use it to duplicate and unfold the an infection.
“You set these two issues [together], and the concept is that now we’ve a protein that detects dsRNA … and it kills the cell,” Kiessig says.
So far, VTose has proven success towards dengue and “a handful of different viruses” in cells — a primary step on a protracted, treacherous path to medical trials and, if it really works there, hospitals.
The Challenges
A number of hurdles stand in the best way of that, not the least of which is whether or not this drug even works at stopping viruses in people in any respect — somewhat than Petri dishes or mice.
“The vast majority of section 1 medicine and therapeutics die in section 1,” Hickman, the DoD senior scientist, says.
There’s good animal fashions for infectious illness — mice and monkeys get contaminated by viruses in a really comparable strategy to people — however many medicine die as a result of they’re merely, and unexpectedly, too poisonous. Proving {that a} broad-spectrum antiviral just isn’t poisonous on the doses it must work can be one hurdle to cross.
Whereas giant molecules are typically much less poisonous, they’re tougher and costly to fabricate, and so they face one other barrier: the regulatory pathway for small molecule antivirals is healthier established. Which means it’s clearer what it’s good to do to persuade the FDA the drug is protected and efficient — what experiments and information it’s good to acquire, how the trials should be designed and run, and so forth.
“They perceive it very well, and the way it’s meant to work from finish to finish,” Kiessig says. There are licensed giant molecule medicine, however the paths should not as nicely trod — and that’s an issue for a small startup.
In fact, all the pieces hinges on a query that may solely be answered by reaching the stage DRACO by no means did: does it work in people?
It’s a radical, formidable concept — the sort that naturally attracts wholesome, warranted skepticism. But when it does, VTose may very well be a strong new weapon for public well being. Kiessig is happy by the prospect.
“The chance right here is super,” Kiessig says. “If we’re profitable, we’ve a possibility to make a change that’s as vital as broad-spectrum antibiotics have been for the world.”

