The ESCRT-III-like protein Vipp1 {couples} filament polymerization with membrane transforming. It assembles planar sheets in addition to 3D rings and helical polymers, all implicated in mitigating plastid-associated membrane stress. The structure of Vipp1 planar sheets and helical polymers stays unknown, as do the geometric adjustments required to transition between polymeric varieties. *
Within the article “Mechanism for Vipp1 spiral formation, ring biogenesis, and membrane restore” Souvik Naskar, Andrea Merino, Javier Espadas, Jayanti Singh, Aurelien Roux, Adai Colom and Harry H. Low present how cyanobacterial Vipp1 assembles into morphologically-related sheets and spirals on membranes in vitro.*
The spirals converge to type a central ring just like these described in membrane budding. Cryo-EM buildings of helical filaments reveal an in depth geometric relationship between Vipp1 helical and planar lattices. Furthermore, the helical buildings reveal how filaments twist—a course of required for Vipp1, and sure different ESCRT-III filaments, to transition between planar and 3D architectures. *
General, the authors’ outcomes present a molecular mannequin for Vipp1 ring biogenesis and a mechanism for Vipp1 membrane stabilization and restore, with implications for different ESCRT-III methods. *
NanoWorld Extremely-Brief Cantilevers USC-F0.3-k0.3 for Excessive-Pace AFM (HS-AFM) with a typical spring fixed of 0.3 N nm−1 and a typical resonance frequency of about 300 kHz have been used for picture acquisition with quick scanning atomic pressure microscopy.*
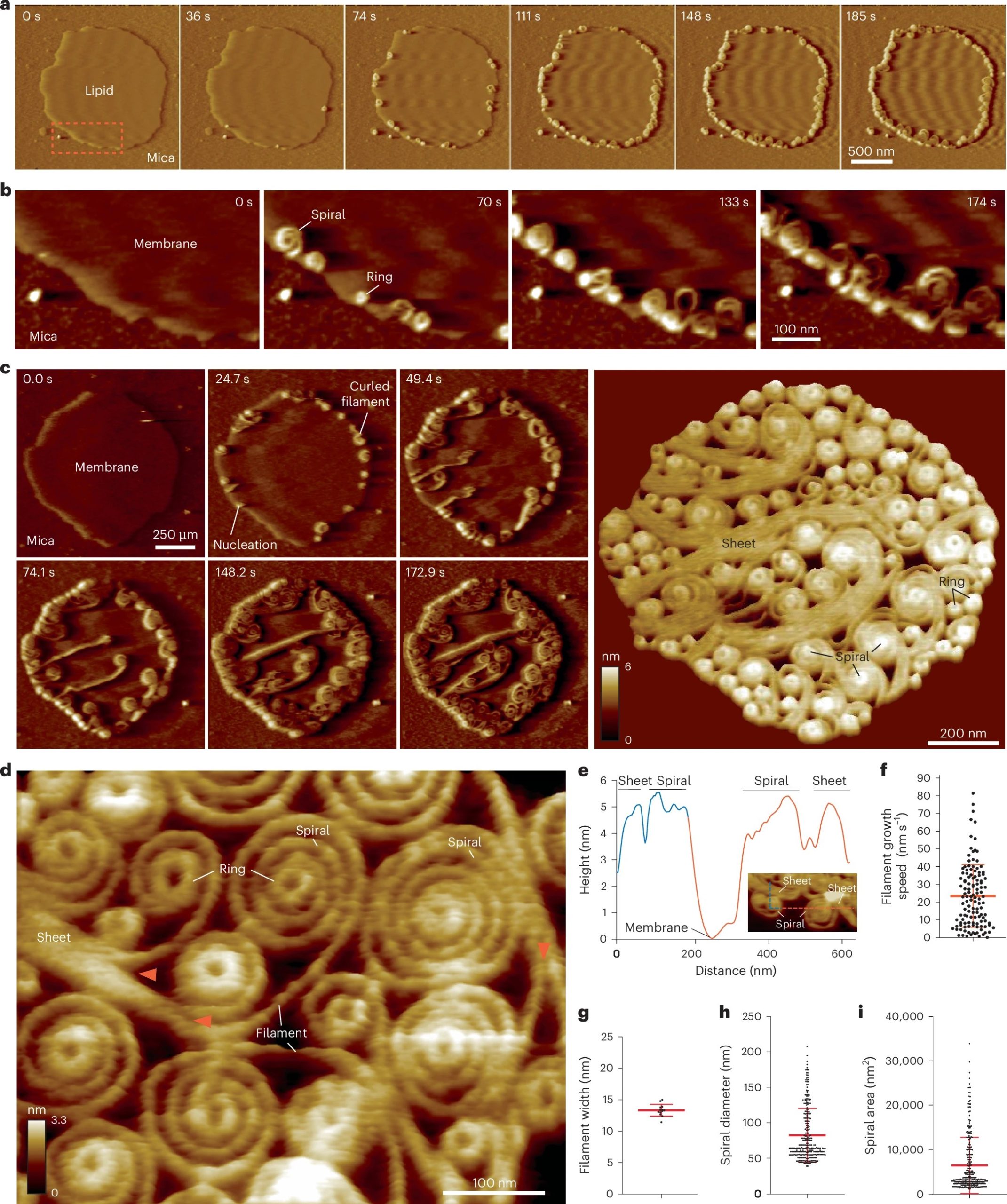
Vipp1 assembles dynamic networks of spirals, rings and sheets on membrane
a, F-AFM part timecourse exhibiting Vipp1 recruitment to the extremely curved fringe of membrane patches. Scan fee, 70 Hz; 256 × 256 pixels. The realm within the dashed field is enlarged in b. b, Spiral and ring formation localized to the membrane edge. Scan fee, 70 Hz; 256 × 256 pixels. c, Left, part timecourse showcasing a dense community of sheets, spirals, and rings that in the end cowl the complete membrane aircraft. Proper, common of six F-AFM top photos. Scan fee, 120 Hz; 256 × 256 pixels. d, Common F-AFM top picture exhibiting Vipp1 sheet, spiral, and ring element. Crimson arrows mark the sheet branching into filaments ~13 nm large. Scan fee, 20 Hz; 256 × 256 pixels. e, Vipp1 sheet and spiral filament top offset from the membrane. f–i, Quantification of Vipp1 filament and spiral traits. n = 124, 13, 278, and 278 impartial measurements for panels f, g, h, and that i, respectively. Error bars present one s.d. of the imply.
*Souvik Naskar, Andrea Merino, Javier Espadas, Jayanti Singh, Aurelien Roux, Adai Colom and Harry H. Low
Mechanism for Vipp1 spiral formation, ring biogenesis, and membrane restore
Nature Structural & Molecular Biology (2024)
DOI: https://doi.org/10.1038/s41594-024-01401-8
Open Entry The article “Mechanism for Vipp1 spiral formation, ring biogenesis, and membrane restore” by Souvik Naskar, Andrea Merino, Javier Espadas, Jayanti Singh, Aurelien Roux, Adai Colom and Harry H. Low is licensed beneath a Artistic Commons Attribution 4.0 Worldwide License, which allows use, sharing, adaptation, distribution and copy in any medium or format, so long as you give applicable credit score to the unique creator(s) and the supply, present a hyperlink to the Artistic Commons license, and point out if adjustments have been made. The pictures or different third occasion materials on this article are included within the article’s Artistic Commons license, until indicated in any other case in a credit score line to the fabric. If materials will not be included within the article’s Artistic Commons license and your meant use will not be permitted by statutory regulation or exceeds the permitted use, you will have to acquire permission immediately from the copyright holder. To view a replica of this license, go to http://creativecommons.org/licenses/by/4.0/.

