When what will not be sufficient
True, generally it’s very important to tell apart between completely different sorts of objects. Is {that a} automobile rushing in direction of me, during which case I’d higher leap out of the way in which? Or is it an enormous Doberman (during which case I’d most likely do the identical)? Typically in actual life although, as a substitute of coarse-grained classification, what is required is fine-grained segmentation.
Zooming in on pictures, we’re not searching for a single label; as a substitute, we wish to classify each pixel in keeping with some criterion:
-
In drugs, we might wish to distinguish between completely different cell sorts, or determine tumors.
-
In numerous earth sciences, satellite tv for pc knowledge are used to phase terrestrial surfaces.
-
To allow use of customized backgrounds, video-conferencing software program has to have the ability to inform foreground from background.
Picture segmentation is a type of supervised studying: Some type of floor reality is required. Right here, it is available in type of a masks – a picture, of spatial decision an identical to that of the enter knowledge, that designates the true class for each pixel. Accordingly, classification loss is calculated pixel-wise; losses are then summed as much as yield an combination for use in optimization.
The “canonical” structure for picture segmentation is U-Web (round since 2015).
U-Web
Right here is the prototypical U-Web, as depicted within the authentic Rönneberger et al. paper (Ronneberger, Fischer, and Brox 2015).
Of this structure, quite a few variants exist. You possibly can use completely different layer sizes, activations, methods to realize downsizing and upsizing, and extra. Nonetheless, there’s one defining attribute: the U-shape, stabilized by the “bridges” crossing over horizontally in any respect ranges.

In a nutshell, the left-hand aspect of the U resembles the convolutional architectures utilized in picture classification. It successively reduces spatial decision. On the identical time, one other dimension – the channels dimension – is used to construct up a hierarchy of options, starting from very fundamental to very specialised.
Not like in classification, nevertheless, the output ought to have the identical spatial decision because the enter. Thus, we have to upsize once more – that is taken care of by the right-hand aspect of the U. However, how are we going to reach at a superb per-pixel classification, now that a lot spatial info has been misplaced?
That is what the “bridges” are for: At every stage, the enter to an upsampling layer is a concatenation of the earlier layer’s output – which went via the entire compression/decompression routine – and a few preserved intermediate illustration from the downsizing section. On this manner, a U-Web structure combines consideration to element with characteristic extraction.
Mind picture segmentation
With U-Web, area applicability is as broad because the structure is versatile. Right here, we wish to detect abnormalities in mind scans. The dataset, utilized in Buda, Saha, and Mazurowski (2019), accommodates MRI pictures along with manually created FLAIR abnormality segmentation masks. It’s out there on Kaggle.
Properly, the paper is accompanied by a GitHub repository. Under, we intently observe (although not precisely replicate) the authors’ preprocessing and knowledge augmentation code.
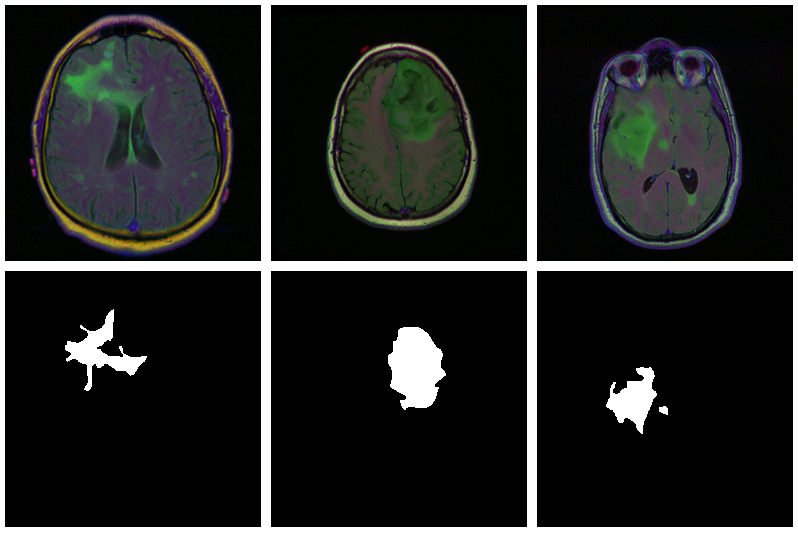
As is commonly the case in medical imaging, there’s notable class imbalance within the knowledge. For each affected person, sections have been taken at a number of positions. (Variety of sections per affected person varies.) Most sections don’t exhibit any lesions; the corresponding masks are coloured black in every single place.
Listed here are three examples the place the masks do point out abnormalities:

Let’s see if we are able to construct a U-Web that generates such masks for us.
Information
Earlier than you begin typing, here’s a Colaboratory pocket book to conveniently observe alongside.
We use pins to acquire the info. Please see this introduction when you haven’t used that bundle earlier than.
The dataset will not be that large – it contains scans from 110 completely different sufferers – so we’ll must do with only a coaching and a validation set. (Don’t do that in actual life, as you’ll inevitably find yourself fine-tuning on the latter.)
train_dir <- "knowledge/mri_train"
valid_dir <- "knowledge/mri_valid"
if(dir.exists(train_dir)) unlink(train_dir, recursive = TRUE, drive = TRUE)
if(dir.exists(valid_dir)) unlink(valid_dir, recursive = TRUE, drive = TRUE)
zip::unzip(recordsdata, exdir = "knowledge")
file.rename("knowledge/kaggle_3m", train_dir)
# it is a duplicate, once more containing kaggle_3m (evidently a packaging error on Kaggle)
# we simply take away it
unlink("knowledge/lgg-mri-segmentation", recursive = TRUE)
dir.create(valid_dir)Of these 110 sufferers, we preserve 30 for validation. Some extra file manipulations, and we’re arrange with a pleasant hierarchical construction, with train_dir and valid_dir holding their per-patient sub-directories, respectively.
valid_indices <- pattern(1:size(sufferers), 30)
sufferers <- checklist.dirs(train_dir, recursive = FALSE)
for (i in valid_indices) {
dir.create(file.path(valid_dir, basename(sufferers[i])))
for (f in checklist.recordsdata(sufferers[i])) {
file.rename(file.path(train_dir, basename(sufferers[i]), f), file.path(valid_dir, basename(sufferers[i]), f))
}
unlink(file.path(train_dir, basename(sufferers[i])), recursive = TRUE)
}We now want a dataset that is aware of what to do with these recordsdata.
Dataset
Like each torch dataset, this one has initialize() and .getitem() strategies. initialize() creates a list of scan and masks file names, for use by .getitem() when it really reads these recordsdata. In distinction to what we’ve seen in earlier posts, although , .getitem() doesn’t merely return input-target pairs so as. As a substitute, each time the parameter random_sampling is true, it can carry out weighted sampling, preferring objects with sizable lesions. This selection might be used for the coaching set, to counter the category imbalance talked about above.
The opposite manner coaching and validation units will differ is use of information augmentation. Coaching pictures/masks could also be flipped, re-sized, and rotated; possibilities and quantities are configurable.
An occasion of brainseg_dataset encapsulates all this performance:
brainseg_dataset <- dataset(
title = "brainseg_dataset",
initialize = operate(img_dir,
augmentation_params = NULL,
random_sampling = FALSE) {
self$pictures <- tibble(
img = grep(
checklist.recordsdata(
img_dir,
full.names = TRUE,
sample = "tif",
recursive = TRUE
),
sample = 'masks',
invert = TRUE,
worth = TRUE
),
masks = grep(
checklist.recordsdata(
img_dir,
full.names = TRUE,
sample = "tif",
recursive = TRUE
),
sample = 'masks',
worth = TRUE
)
)
self$slice_weights <- self$calc_slice_weights(self$pictures$masks)
self$augmentation_params <- augmentation_params
self$random_sampling <- random_sampling
},
.getitem = operate(i) {
index <-
if (self$random_sampling == TRUE)
pattern(1:self$.size(), 1, prob = self$slice_weights)
else
i
img <- self$pictures$img[index] %>%
image_read() %>%
transform_to_tensor()
masks <- self$pictures$masks[index] %>%
image_read() %>%
transform_to_tensor() %>%
transform_rgb_to_grayscale() %>%
torch_unsqueeze(1)
img <- self$min_max_scale(img)
if (!is.null(self$augmentation_params)) {
scale_param <- self$augmentation_params[1]
c(img, masks) %<-% self$resize(img, masks, scale_param)
rot_param <- self$augmentation_params[2]
c(img, masks) %<-% self$rotate(img, masks, rot_param)
flip_param <- self$augmentation_params[3]
c(img, masks) %<-% self$flip(img, masks, flip_param)
}
checklist(img = img, masks = masks)
},
.size = operate() {
nrow(self$pictures)
},
calc_slice_weights = operate(masks) {
weights <- map_dbl(masks, operate(m) {
img <-
as.integer(magick::image_data(image_read(m), channels = "grey"))
sum(img / 255)
})
sum_weights <- sum(weights)
num_weights <- size(weights)
weights <- weights %>% map_dbl(operate(w) {
w <- (w + sum_weights * 0.1 / num_weights) / (sum_weights * 1.1)
})
weights
},
min_max_scale = operate(x) {
min = x$min()$merchandise()
max = x$max()$merchandise()
x$clamp_(min = min, max = max)
x$add_(-min)$div_(max - min + 1e-5)
x
},
resize = operate(img, masks, scale_param) {
img_size <- dim(img)[2]
rnd_scale <- runif(1, 1 - scale_param, 1 + scale_param)
img <- transform_resize(img, dimension = rnd_scale * img_size)
masks <- transform_resize(masks, dimension = rnd_scale * img_size)
diff <- dim(img)[2] - img_size
if (diff > 0) {
high <- ceiling(diff / 2)
left <- ceiling(diff / 2)
img <- transform_crop(img, high, left, img_size, img_size)
masks <- transform_crop(masks, high, left, img_size, img_size)
} else {
img <- transform_pad(img,
padding = -c(
ceiling(diff / 2),
flooring(diff / 2),
ceiling(diff / 2),
flooring(diff / 2)
))
masks <- transform_pad(masks, padding = -c(
ceiling(diff / 2),
flooring(diff /
2),
ceiling(diff /
2),
flooring(diff /
2)
))
}
checklist(img, masks)
},
rotate = operate(img, masks, rot_param) {
rnd_rot <- runif(1, 1 - rot_param, 1 + rot_param)
img <- transform_rotate(img, angle = rnd_rot)
masks <- transform_rotate(masks, angle = rnd_rot)
checklist(img, masks)
},
flip = operate(img, masks, flip_param) {
rnd_flip <- runif(1)
if (rnd_flip > flip_param) {
img <- transform_hflip(img)
masks <- transform_hflip(masks)
}
checklist(img, masks)
}
)After instantiation, we see we now have 2977 coaching pairs and 952 validation pairs, respectively:
As a correctness examine, let’s plot a picture and related masks:

With torch, it’s simple to examine what occurs whenever you change augmentation-related parameters. We simply decide a pair from the validation set, which has not had any augmentation utilized as but, and name valid_ds$<augmentation_func()> instantly. Only for enjoyable, let’s use extra “excessive” parameters right here than we do in precise coaching. (Precise coaching makes use of the settings from Mateusz’ GitHub repository, which we assume have been rigorously chosen for optimum efficiency.)
img_and_mask <- valid_ds[77]
img <- img_and_mask[[1]]
masks <- img_and_mask[[2]]
imgs <- map (1:24, operate(i) {
# scale issue; train_ds actually makes use of 0.05
c(img, masks) %<-% valid_ds$resize(img, masks, 0.2)
c(img, masks) %<-% valid_ds$flip(img, masks, 0.5)
# rotation angle; train_ds actually makes use of 15
c(img, masks) %<-% valid_ds$rotate(img, masks, 90)
img %>%
transform_rgb_to_grayscale() %>%
as.array() %>%
as_tibble() %>%
rowid_to_column(var = "Y") %>%
collect(key = "X", worth = "worth", -Y) %>%
mutate(X = as.numeric(gsub("V", "", X))) %>%
ggplot(aes(X, Y, fill = worth)) +
geom_raster() +
theme_void() +
theme(legend.place = "none") +
theme(facet.ratio = 1)
})
plot_grid(plotlist = imgs, nrow = 4)
Now we nonetheless want the info loaders, after which, nothing retains us from continuing to the following large process: constructing the mannequin.
batch_size <- 4
train_dl <- dataloader(train_ds, batch_size)
valid_dl <- dataloader(valid_ds, batch_size)Mannequin
Our mannequin properly illustrates the type of modular code that comes “naturally” with torch. We strategy issues top-down, beginning with the U-Web container itself.
unet takes care of the worldwide composition – how far “down” will we go, shrinking the picture whereas incrementing the variety of filters, after which how will we go “up” once more?
Importantly, it is usually within the system’s reminiscence. In ahead(), it retains monitor of layer outputs seen going “down,” to be added again in going “up.”
unet <- nn_module(
"unet",
initialize = operate(channels_in = 3,
n_classes = 1,
depth = 5,
n_filters = 6) {
self$down_path <- nn_module_list()
prev_channels <- channels_in
for (i in 1:depth) {
self$down_path$append(down_block(prev_channels, 2 ^ (n_filters + i - 1)))
prev_channels <- 2 ^ (n_filters + i -1)
}
self$up_path <- nn_module_list()
for (i in ((depth - 1):1)) {
self$up_path$append(up_block(prev_channels, 2 ^ (n_filters + i - 1)))
prev_channels <- 2 ^ (n_filters + i - 1)
}
self$final = nn_conv2d(prev_channels, n_classes, kernel_size = 1)
},
ahead = operate(x) {
blocks <- checklist()
for (i in 1:size(self$down_path)) {
x <- self$down_path[[i]](x)
if (i != size(self$down_path)) {
blocks <- c(blocks, x)
x <- nnf_max_pool2d(x, 2)
}
}
for (i in 1:size(self$up_path)) {
x <- self$up_path[[i]](x, blocks[[length(blocks) - i + 1]]$to(system = system))
}
torch_sigmoid(self$final(x))
}
)unet delegates to 2 containers slightly below it within the hierarchy: down_block and up_block. Whereas down_block is “simply” there for aesthetic causes (it instantly delegates to its personal workhorse, conv_block), in up_block we see the U-Web “bridges” in motion.
down_block <- nn_module(
"down_block",
initialize = operate(in_size, out_size) {
self$conv_block <- conv_block(in_size, out_size)
},
ahead = operate(x) {
self$conv_block(x)
}
)
up_block <- nn_module(
"up_block",
initialize = operate(in_size, out_size) {
self$up = nn_conv_transpose2d(in_size,
out_size,
kernel_size = 2,
stride = 2)
self$conv_block = conv_block(in_size, out_size)
},
ahead = operate(x, bridge) {
up <- self$up(x)
torch_cat(checklist(up, bridge), 2) %>%
self$conv_block()
}
)Lastly, a conv_block is a sequential construction containing convolutional, ReLU, and dropout layers.
conv_block <- nn_module(
"conv_block",
initialize = operate(in_size, out_size) {
self$conv_block <- nn_sequential(
nn_conv2d(in_size, out_size, kernel_size = 3, padding = 1),
nn_relu(),
nn_dropout(0.6),
nn_conv2d(out_size, out_size, kernel_size = 3, padding = 1),
nn_relu()
)
},
ahead = operate(x){
self$conv_block(x)
}
)Now instantiate the mannequin, and probably, transfer it to the GPU:
system <- torch_device(if(cuda_is_available()) "cuda" else "cpu")
mannequin <- unet(depth = 5)$to(system = system)Optimization
We prepare our mannequin with a mix of cross entropy and cube loss.
The latter, although not shipped with torch, could also be applied manually:
calc_dice_loss <- operate(y_pred, y_true) {
clean <- 1
y_pred <- y_pred$view(-1)
y_true <- y_true$view(-1)
intersection <- (y_pred * y_true)$sum()
1 - ((2 * intersection + clean) / (y_pred$sum() + y_true$sum() + clean))
}
dice_weight <- 0.3Optimization makes use of stochastic gradient descent (SGD), along with the one-cycle studying price scheduler launched within the context of picture classification with torch.
optimizer <- optim_sgd(mannequin$parameters, lr = 0.1, momentum = 0.9)
num_epochs <- 20
scheduler <- lr_one_cycle(
optimizer,
max_lr = 0.1,
steps_per_epoch = size(train_dl),
epochs = num_epochs
)Coaching
The coaching loop then follows the same old scheme. One factor to notice: Each epoch, we save the mannequin (utilizing torch_save()), so we are able to later decide the very best one, ought to efficiency have degraded thereafter.
train_batch <- operate(b) {
optimizer$zero_grad()
output <- mannequin(b[[1]]$to(system = system))
goal <- b[[2]]$to(system = system)
bce_loss <- nnf_binary_cross_entropy(output, goal)
dice_loss <- calc_dice_loss(output, goal)
loss <- dice_weight * dice_loss + (1 - dice_weight) * bce_loss
loss$backward()
optimizer$step()
scheduler$step()
checklist(bce_loss$merchandise(), dice_loss$merchandise(), loss$merchandise())
}
valid_batch <- operate(b) {
output <- mannequin(b[[1]]$to(system = system))
goal <- b[[2]]$to(system = system)
bce_loss <- nnf_binary_cross_entropy(output, goal)
dice_loss <- calc_dice_loss(output, goal)
loss <- dice_weight * dice_loss + (1 - dice_weight) * bce_loss
checklist(bce_loss$merchandise(), dice_loss$merchandise(), loss$merchandise())
}
for (epoch in 1:num_epochs) {
mannequin$prepare()
train_bce <- c()
train_dice <- c()
train_loss <- c()
coro::loop(for (b in train_dl) {
c(bce_loss, dice_loss, loss) %<-% train_batch(b)
train_bce <- c(train_bce, bce_loss)
train_dice <- c(train_dice, dice_loss)
train_loss <- c(train_loss, loss)
})
torch_save(mannequin, paste0("model_", epoch, ".pt"))
cat(sprintf("nEpoch %d, coaching: loss:%3f, bce: %3f, cube: %3fn",
epoch, imply(train_loss), imply(train_bce), imply(train_dice)))
mannequin$eval()
valid_bce <- c()
valid_dice <- c()
valid_loss <- c()
i <- 0
coro::loop(for (b in tvalid_dl) {
i <<- i + 1
c(bce_loss, dice_loss, loss) %<-% valid_batch(b)
valid_bce <- c(valid_bce, bce_loss)
valid_dice <- c(valid_dice, dice_loss)
valid_loss <- c(valid_loss, loss)
})
cat(sprintf("nEpoch %d, validation: loss:%3f, bce: %3f, cube: %3fn",
epoch, imply(valid_loss), imply(valid_bce), imply(valid_dice)))
}Epoch 1, coaching: loss:0.304232, bce: 0.148578, cube: 0.667423
Epoch 1, validation: loss:0.333961, bce: 0.127171, cube: 0.816471
Epoch 2, coaching: loss:0.194665, bce: 0.101973, cube: 0.410945
Epoch 2, validation: loss:0.341121, bce: 0.117465, cube: 0.862983
[...]
Epoch 19, coaching: loss:0.073863, bce: 0.038559, cube: 0.156236
Epoch 19, validation: loss:0.302878, bce: 0.109721, cube: 0.753577
Epoch 20, coaching: loss:0.070621, bce: 0.036578, cube: 0.150055
Epoch 20, validation: loss:0.295852, bce: 0.101750, cube: 0.748757Analysis
On this run, it’s the last mannequin that performs finest on the validation set. Nonetheless, we’d like to indicate load a saved mannequin, utilizing torch_load() .
As soon as loaded, put the mannequin into eval mode:
saved_model <- torch_load("model_20.pt")
mannequin <- saved_model
mannequin$eval()Now, since we don’t have a separate take a look at set, we already know the typical out-of-sample metrics; however ultimately, what we care about are the generated masks. Let’s view some, displaying floor reality and MRI scans for comparability.
# with out random sampling, we would primarily see lesion-free patches
eval_ds <- brainseg_dataset(valid_dir, augmentation_params = NULL, random_sampling = TRUE)
eval_dl <- dataloader(eval_ds, batch_size = 8)
batch <- eval_dl %>% dataloader_make_iter() %>% dataloader_next()
par(mfcol = c(3, 8), mar = c(0, 1, 0, 1))
for (i in 1:8) {
img <- batch[[1]][i, .., drop = FALSE]
inferred_mask <- mannequin(img$to(system = system))
true_mask <- batch[[2]][i, .., drop = FALSE]$to(system = system)
bce <- nnf_binary_cross_entropy(inferred_mask, true_mask)$to(system = "cpu") %>%
as.numeric()
dc <- calc_dice_loss(inferred_mask, true_mask)$to(system = "cpu") %>% as.numeric()
cat(sprintf("nSample %d, bce: %3f, cube: %3fn", i, bce, dc))
inferred_mask <- inferred_mask$to(system = "cpu") %>% as.array() %>% .[1, 1, , ]
inferred_mask <- ifelse(inferred_mask > 0.5, 1, 0)
img[1, 1, ,] %>% as.array() %>% as.raster() %>% plot()
true_mask$to(system = "cpu")[1, 1, ,] %>% as.array() %>% as.raster() %>% plot()
inferred_mask %>% as.raster() %>% plot()
}We additionally print the person cross entropy and cube losses; relating these to the generated masks may yield helpful info for mannequin tuning.
Pattern 1, bce: 0.088406, cube: 0.387786}
Pattern 2, bce: 0.026839, cube: 0.205724
Pattern 3, bce: 0.042575, cube: 0.187884
Pattern 4, bce: 0.094989, cube: 0.273895
Pattern 5, bce: 0.026839, cube: 0.205724
Pattern 6, bce: 0.020917, cube: 0.139484
Pattern 7, bce: 0.094989, cube: 0.273895
Pattern 8, bce: 2.310956, cube: 0.999824
Whereas removed from excellent, most of those masks aren’t that unhealthy – a pleasant consequence given the small dataset!
Wrapup
This has been our most complicated torch publish to this point; nevertheless, we hope you’ve discovered the time nicely spent. For one, amongst functions of deep studying, medical picture segmentation stands out as extremely societally helpful. Secondly, U-Web-like architectures are employed in lots of different areas. And at last, we as soon as extra noticed torch’s flexibility and intuitive habits in motion.
Thanks for studying!