A novel antibiotic named Clovibactin, extracted from beforehand uncultivable micro organism, has been discovered efficient in opposition to dangerous micro organism, together with multi-resistant “superbugs”. Clovibactin’s distinctive mechanism of concentrating on a number of important precursor molecules within the bacterial cell wall makes it troublesome for micro organism to develop into immune to it.
A brand new highly effective antibiotic, remoted from beforehand unstudied micro organism, reveals promise in tackling dangerous micro organism, together with the multi-resistant ‘superbugs’. Dubbed “Clovibactin,” this antibiotic destroys micro organism in an uncommon manner, making it harder for micro organism to develop any resistance in opposition to it.
Researchers from Utrecht College, Bonn College (Germany), the German Middle for An infection Analysis (DZIF), Northeastern College of Boston (USA), and the corporate NovoBiotic Prescription drugs (Cambridge, USA) now share the invention of Clovibactin and its killing mechanism within the scientific journal Cell.
There’s an pressing want for brand new antibiotics
Antimicrobial resistance is a serious drawback for human well being and researchers worldwide are in search of new options. “We urgently want new antibiotics to fight micro organism that develop into more and more immune to most clinically used antibiotics,” says Dr. Markus Weingarth, a researcher from the Chemistry Division of Utrecht College.
“Clovibactin is totally different,” says Weingarth. “Since Clovibactin was remoted from micro organism that would not be grown earlier than, pathogenic micro organism haven’t seen such an antibiotic earlier than and had no time to develop resistance.”
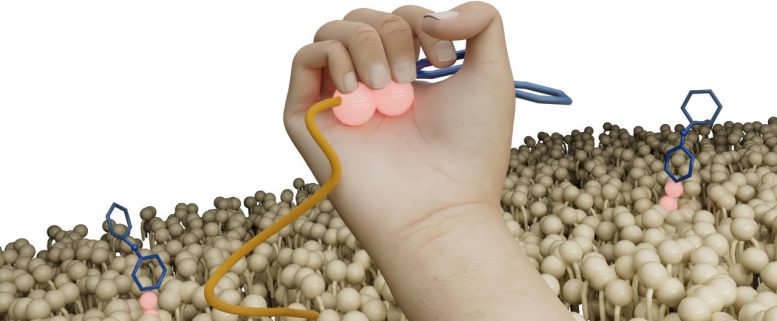
The newly found antibiotic Clovibactin makes use of an unsual cage-like binding motif to tightly wrap round particular lipids in bacterial cell membranes. Credit score: Markus Weingarth
Antibiotic from bacterial darkish matter
Clovibactin was found by NovoBiotic Prescription drugs, a small US-based early-stage firm, and microbiologist Prof. Kim Lewis from Northeastern College, Boston. Earlier, they developed a tool that enables the expansion of ‘bacterial darkish matter’, that are so-called unculturable micro organism. Intriguingly, 99% of all micro organism are ‘unculturable’ and couldn’t be grown in laboratories beforehand, therefore they may not be mined for novel antibiotics. Utilizing the gadget, known as iCHip, the US researchers found Clovibactin in a bacterium remoted from sandy soil from North Carolina: E. terrae ssp. Carolina.
Within the joint Cell publication, NovoBiotic Prescription drugs reveals that Clovibactin efficiently assaults a broad spectrum of bacterial pathogens. It was additionally efficiently used to deal with mice contaminated with the superbug Staphylococcus aureus.
A broad goal spectrum
Clovibactin seems to have an uncommon killing mechanism. It targets not only one, however three totally different precursor molecules which might be all important for the development of the cell wall, an envelope-like construction that surrounds micro organism. This was found by the group of Prof. Tanja Schneider from the College of Bonn in Germany, one of many Cell paper’s co-authors.
Schneider: “The multi-target assault mechanism of Clovibactin blocks bacterial cell wall synthesis concurrently at totally different positions. This improves the drug’s exercise and considerably will increase its robustness to resistance growth.”
A cage-like construction
How precisely Clovibactin blocks the synthesis of the bacterial cell wall was unraveled by the workforce of Dr. Markus Weingarth from Utrecht College. They used a particular approach known as solid-state nuclear magnetic resonance (NMR) that allowed them to check Clovibactin’s mechanism underneath related situations as in micro organism.
“Clovibactin wraps across the pyrophosphate like a tightly becoming glove. Like a cage that encloses its goal” says Weingarth. That is what offers Clovibactin its title, which is derived from the Greek phrase “Klouvi”, which suggests cage. The outstanding facet of Clovibactin’s mechanism is that it solely binds to the immutable pyrophosphate that’s frequent to cell wall precursors, but it surely ignores that variable sugar-peptide a part of the targets. “As Clovibactin solely binds to the immutable, conserved a part of its targets, micro organism may have a a lot more durable time creating any resistance in opposition to it. In truth, we didn’t observe any resistance to Clovibactin in our research.”
Fibrils seize the targets
Clovibactin can do much more. Upon binding the goal molecules, it self-assembles into massive fibrils on the floor of bacterial membranes. These fibrils are secure for a very long time and thereby be sure that the goal molecules stay sequestered for so long as essential to kill micro organism.
“Since these fibrils solely kind on bacterial membranes and never on human membranes, they’re presumably additionally the explanation why Clovibactin selectively damages bacterial cells however isn’t poisonous to human cells,” says Weingarth. “Clovibactin therefore has potential for the design of improved therapeutics that kill bacterial pathogens with out resistance growth.”
Reference: “An antibiotic from an uncultured bacterium binds to an immutable goal” by Rhythm Shukla, Aaron J. Peoples, Kevin C. Ludwig, Sourav Maity, Maik G.N. Derks, Stefania De Benedetti, Annika M. Krueger, Bram J.A. Vermeulen, Theresa Harbig, Francesca Lavore, Raj Kumar, Rodrigo V. Honorato, Fabian Grein, Kay Nieselt, Yangping Liu, Alexandre M.J.J. Bonvin, Marc Baldus, Ulrich Kubitscheck, Eefjan Breukink, Catherine Achorn and Markus Weingarth, 22 August 2023, Cell.
DOI: 10.1016/j.cell.2023.07.038